PSP Platforms – Go External or DIY?

Things to Keep in Mind When Setting Up Your Patient Support Program
Since DKSH launched PSPhere Lite+ as part of the PSPhere product portfolio in September 2021, we have rapidly rolled out Patient Access Programs (PAPs) across Asia Pacific markets. We identified the need for a standard and cost-effective solution for pharmaceutical companies to implement their Patient Support Programs (PSP). While our hypothesis is proving right, there is more to do in engaging patients and in helping them achieve better healthcare outcomes using PSPhere, our patient benefits and relationship management platform, built to solve the problem of administering beyond-the-pill solutions for patients.
We have observed a trend with a few large multinational pharmaceuticals’ companies developing their platforms to manage PSPs (not developed in-house, but with a technology partner; like drug development works with biotech players) in the rapidly evolving beyond-the-pill solutions space. Whilst the intention of developing and deploying these platforms is usually to establish controls, compliance mechanisms, achieve harmonization for PSPs across markets and standardize reporting, their use in the real world, when used by patients, doctors, and other stakeholders can lead to fragmented user experience, data security, and privacy challenges. (1)
At a market-level, multinational pharmaceutical companies are under growing pressure to deliver greater financial performance since a market is ultimately where the “rubber hits the road”. APAC markets are classified high on every Chief Digital Officer’s (CDO) Digital Maturity Index. Malaysia and Australia would be short-listed for prestigious pilots on the latest global PSP platforms commissioned on Salesforce’s Health Cloud, Deloitte’s ConvergeHEALTH Connect™, or Accenture’s INTIENT Patient. (2a,2b) Being selected for such pilots allows markets to stand out from others and display their capabilities. This brings the added benefit of not having to pay third-party vendors for the development of the PSP platform since the bulk of the costs have been covered by the CDO or above-market structure.
As we take ethics and compliance involved in running PSPs very seriously here are some critical success factors on if and how your in-house platforms can be used in the management of PSPs in Asia Pacific, and how you can manage the downside risks by considering our key arguments.
Critical Success Factors in Running or Starting Your PSP:
- User Experience
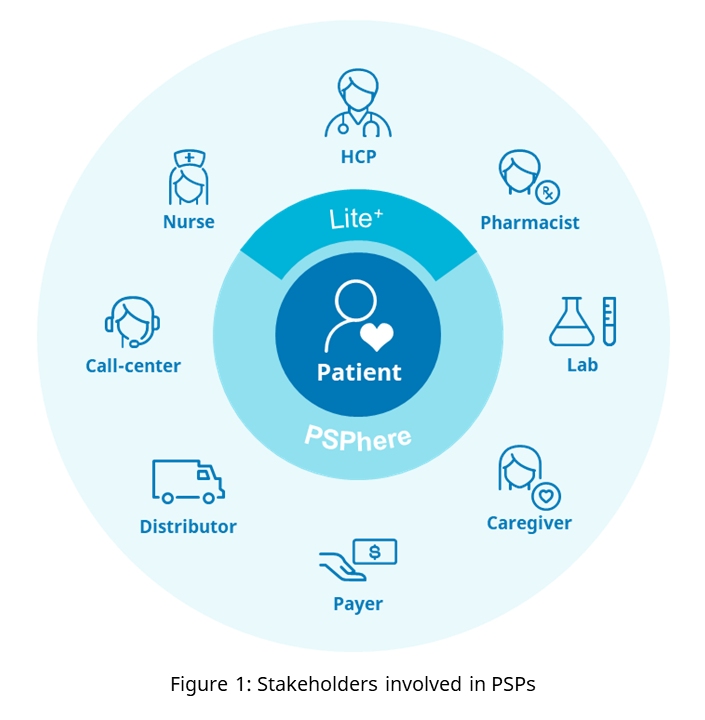
Exceptional user experience is paramount for all stakeholders (Figure 1).
The interaction with a platform must be smooth and focused on the needs of the user, intuitive and never painstaking. To achieve a constant improvement in experience, usage statistics from millions of patients should be analyzed to improve the process and overall user experience. Imagine the situation that existed in many companies where one had to log in to multiple applications with unique usernames and password combinations. The time spent remembering and resetting passwords led to a poor user experience and wasted employee time. Many companies have since improved the situation by introducing single sign-on (SSO) to ensure a better user experience for employees navigating multiple applications while completing their work.
Now apply this to patient programs. In a world where most pharmaceutical brands have a PSP, and each company deploys its PSP application to manage these programs in the real world (referred to as “App” in Figure 2a &b). This means different applications for doctors and patients to access each company/brand’s patient programs. Imagine the username and password burden for patients, doctors, pharmacists, and the subsequent inertia.
Referencing precedents set in other industries, the ideal solution to this problem would involve combining all the doctors and patients onto a platform to ensure the best possible user experience (Figure 2a&b).
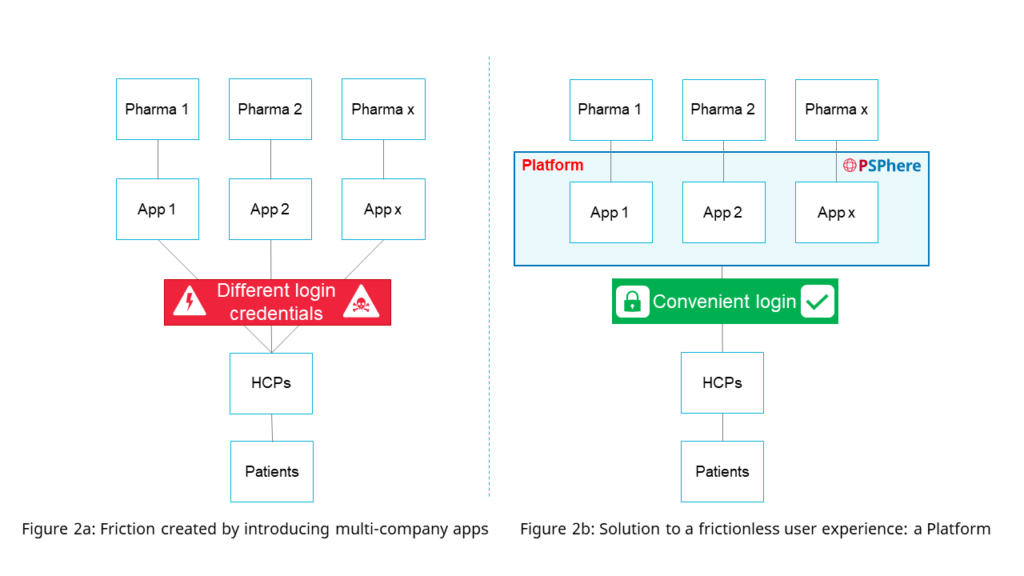
A single company/brand approach per patient program will hinder continuous improvements. In contrast, consolidation of multiple company’s and brand’s patient programs on a platform will lead to greater user numbers, better analytics, and ultimately an improved user experience for all its users.
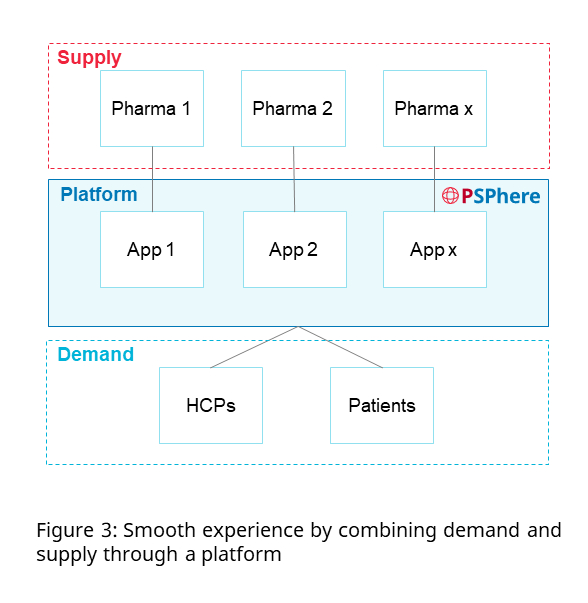
Like other marketplace platforms such as Lazada, a PSP platform brings supply (pharmaceutical brands) to demand (HCPs and patients) ensuring that pharmaceutical companies’ brands are available at the right place. The platform unites the demand and supply side by having patients, HCPs and the PSP programs of pharma clients all sharing the same space, simplifying the experience, and leading to greater adoption compared to the friction created by a single company approach such as a company-branded store (Figure 3).
2. Data Privacy, Data Security and Pharmaceutical Legislation
While user experience is important, data privacy and security are paramount to maintaining trust with patients and healthcare stakeholders. Notable recent ransomware attacks (WannaCry, NotPetya, and REvil) have made everyone aware of the damages attacks on systems can have.
Financial damages reaching from a couple of millions up to USD 1.3 billion in the case of Merck Sharp & Dohme (MSD) (3,4) or the damages in trust and reputation in the case of the SingHealth data breach where data of 1.5 million Singaporean patients was stolen (5,6). As PwC noted in its lessons on the SingHealth attack, many companies still have a mindset of “it will never happen to us”.
Absolute security does not exist in the world, neither on systems nor in the real world. Outsourcing your PSP, including the development and operation of the platform, to a partner and therefore independently assessing them, helps to improve security, as one will be more diligent with an external party than looking at internal gaps. This will help to improve your exposure and ensures the partners readiness for a potential cyber-attack.
The answer to restore trust in applications and build a platform for patient programs is three-fold:
a. Data Privacy
Any platform must consider data privacy at its core. As PSP platforms hold sensitive health/medical (in some cases financial) data, the protection of this data must be managed in accordance with the highest international standards like HIPAA or GDPR.
Patient interactions must always start with capturing informed patient consent to ensure that patients understand what data of theirs is collected, where it will be stored, and for how long as well as for what purpose a company will use it. Finally, patients must be aware and understand how they can exercise their right to privacy such as their right to understand what data a company keeps or their right to be forgotten.
b. Data Security
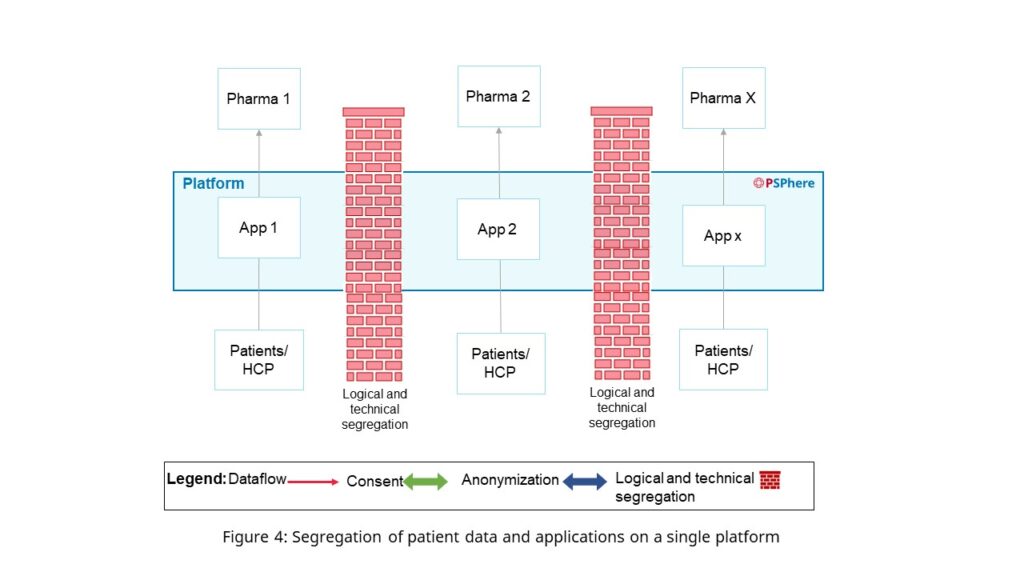
A PSP provider has to take utmost care to protect their platform by continuously incorporating best industry data security practices, ensuring independent verification, for example, through certification and having rigorous and regular audit programs to ensure up-to-date security. Important for a service provider and their platform managing multiple brands and programs in a pharmaceutical company’s portfolio is the distinct segregation of applications and the corresponding patient data to protect not only from unwanted external access but also from contamination by competing brands’ data due to the lack of patient consent (Figure 4).
Repeated audits by a spectrum of pharmaceuticals companies can help a service provider to enhance data transparency and traceability. Audits give reassurance to companies that PSPs are carried out according to standard operating procedures (SOPs) signed off by compliance and data privacy functions of both parties.
We would never recommend audit shortcuts and we recommend improvements to audits by assuring a best-in-class audit experience while improving cost-effectiveness likethrough e-audits to save time and costs. A PSP service provider must work closely with their partners and implement corrective and preventive improvements to ensure a high level of security.
A platform audited by a multitude of companies and which is observed by many eyes will ensure a different level of security than a platform used by a single company.
c. Pharmaceutical Legislation
Another critical aspect we have observed is anchored in legislation for the pharmaceutical industry around Asia. Pharmaceutical companies should not have access to identifiable personal information of patients. When building an in-house platform this means a pharmaceuticals company must lock themselves out of their platform as it will hold identifiable personal patient information. This means that companies need to find a third-party PSP service provider to operate their in-house platform and rely on the provider in audits. This segregates the platform design, knowledge, and operations, and ultimately leads to audit findings.
The way forward for pharmaceuticals company lies in using a service provider with a proprietary PSP platform so that sensitive patient data can be managed with the appropriate controls. The relationship between a pharmaceutical company and a PSP provider should be maintained at arm’s length with an oversight capacity for the former, as the execution of the PSP is not a core competency of pharma companies.
The PSP provider therefore must manage the sensitivity around data and must ensure they can anonymize patient data. Ideally, this happens in real-time to ensure a relevant and timely flow of information back to decision-makers.
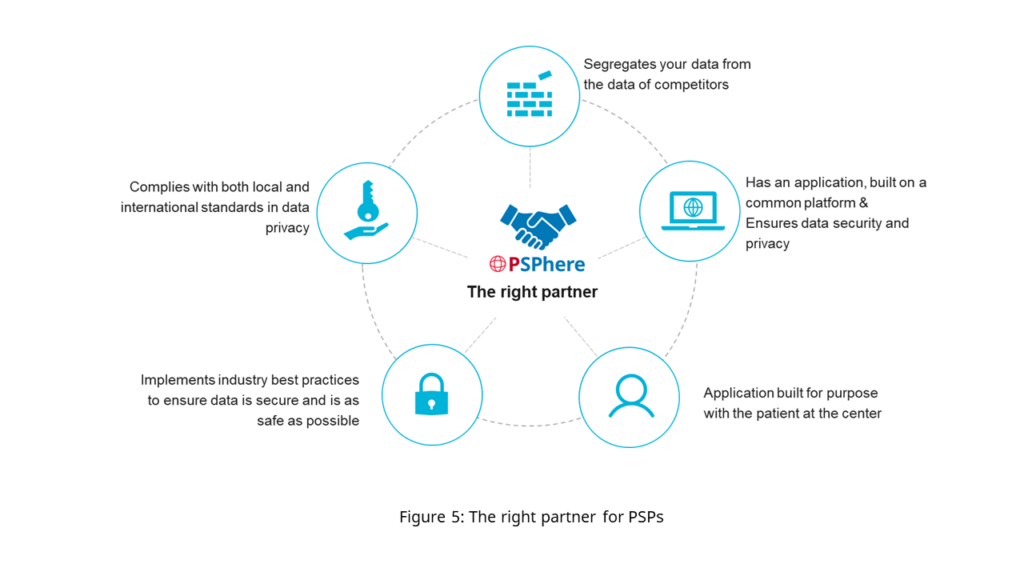
Figure 5 outlines key considerations for companies to identify the right partner to run PSPs.
3. Cost Increase
If multiple pharma companies all bring their platform to a service provider, the service provider will inevitably see an increase in operating cost, as staff must be trained on each of the pharma companies’ in-house platforms. This in turn will lead to an exponential cost increase for each pharma company, as the PSP service provider cannot pass on benefits of scale due to the complexity of managing various platforms. Hence, from a cost perspective, it makes more sense to utilize a provider, with a platform, operated by experienced and trained staff, to ensure a cost-effective deployment of the PSP.
4. Scalability
Given that most pharma companies operate across multiple markets, a platform tailored to programs according to the needs of local stakeholders, ensuring the best possible user experience, while still maintaining consistency can be scaled across other markets. A single platform helps to achieve scalability at a cost advantage, as existing processes do not have to be redeveloped. A single platform ensures consistent protocols, compliance checks and data capture, which leads to consistent reporting and comparable insights across markets. When data is collected in an anonymized and standardized fashion, it unlocks new paths of decision-making that were not obtainable otherwise, and leads to improved patient engagement, access to medication, and ultimately better patient outcomes and a more sustainable business.
Our Recommendation for the Role of Your In-House Platform
PSPs can be a source of competitive advantage because your assets are differentiated along the dimensions of the challenges patients face throughout their healthcare journey (usually access, adherence, and convenience). PSPs are carefully designed by your local teams and us, to ensure that the operational realities involving workflows, controls and user experience are optimized for continuous patient engagement, so that long-term objectives to improve patient outcomes can be met.
Based on extensive operational experience in PSP, we believe that, for large multinational pharmaceutical companies, the cost, and risks of deploying their PSP platform will likely outweigh the benefits.
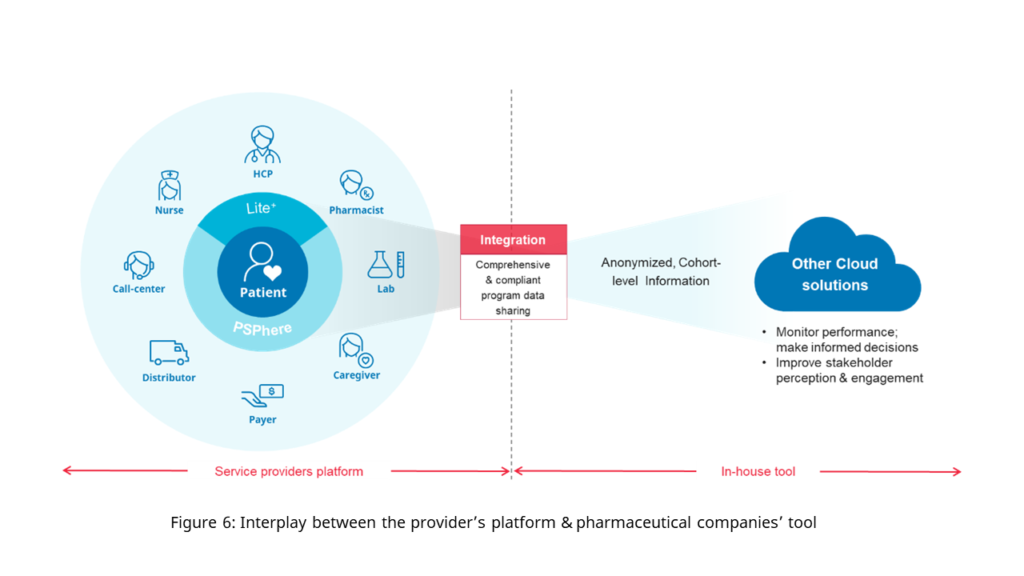
Our recommendation does not mean that your platform becomes redundant. Your in-house platform becomes a tool, which will be used to receive data and insights, to make regular business decisions and maintain oversight. Working with partners such as DKSH to run your PSPs using PSPhere means that your tool can connect directly to the data using an application programming interface (API). This will allow you to focus on making decisions around your core areas of expertise – optimizing selling & marketing, handling medical affairs, and ensuring market access, whilst we run the entirety of your programs and give you the anonymized data in the form and frequency you want it in (figure 6).
Sources:
- Patient Support Programs: what are they and what do they offer?
- Elevate the Digital Patient Experience
- Powering patient-first solutions
- Merck Cyberattack’s $1.3 Billion Question: Was It an Act of War?
- One Year After NotPetya Cyberattack, Firms Wrestle With Recovery Costs
- Singapore’s Prime Minister Among 1.5 Million Affected by Cyberattack
- Key lessons from the Public Report of the Committee of Inquiry (COI) on SingHealth cyber attack